Welcome to the PROMPT Study! PROMPT stands for PONV Rescue Outcomes after AMisulPride Treatment. PROMPT is a registry-based study that will collect and analyze real-world evidence on the presentation, evaluation, and rescue treatment of post-operative nausea and vomiting.
PROMPT is managed through the Perioperative and Anesthesia Care Explorer (PACE) Registry, and is powered by ArborMetrix.
-800x533.jpeg)
The PROMPT Study is a multicenter matched cohort analysis that will compare patient outcomes before and after introduction of intravenous amisulpride as rescue treatment in the post-anesthesia care unit. It has several aims:
The Perioperative and Anesthesia Care Explorer (PACE) Registry is home to the PROMPT Study. Participating sites will use PACE to participate in the PROMPT Study and analyze and understand their own outcomes.
Data from the PROMPT Study will be collected from de-identified electronic health records into the PACE Registry. In the case of the PROMPT Study, the PACE Registry will:
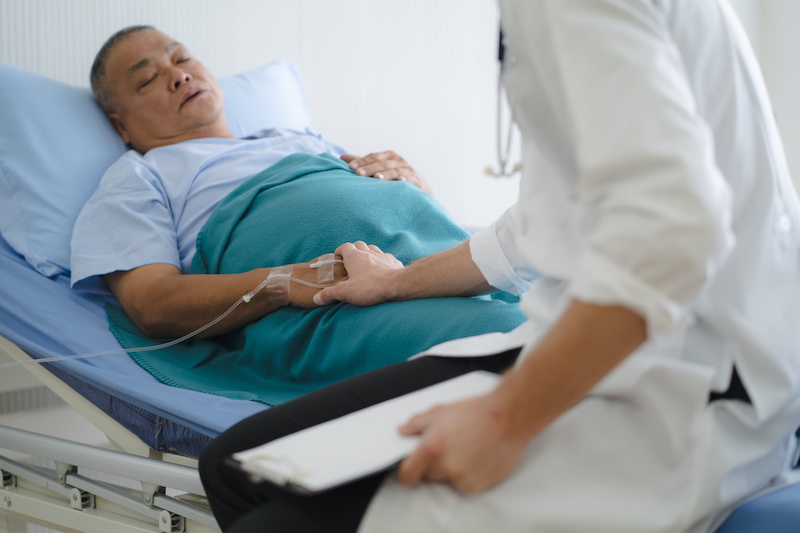
The PROMPT Study is filling the need for large, real-world observational studies in perioperative medicine. This study will help answer key questions as new treatments for PONV become available.
The PROMPT Study will include academic and community hospitals and health systems across the United States. It will collect and analyze real-world data on the presentation, evaluation, and rescue treatment of PONV.
Participating sites and researchers will use the PACE Registry to investigate and publish key outcomes and trends to advance quality and safety.
-800x533.jpeg)

Sites that participate in the PROMPT Study will use the PACE Registry to analyze and understand their own outcomes. They will:
Data for the PROMPT Study will be collected from de-identified electronic health records into the PACE Registry, and compiled and analyzed by an independent data science company, ArborMetrix.
©2026 ArborMetrix. All rights reserved.